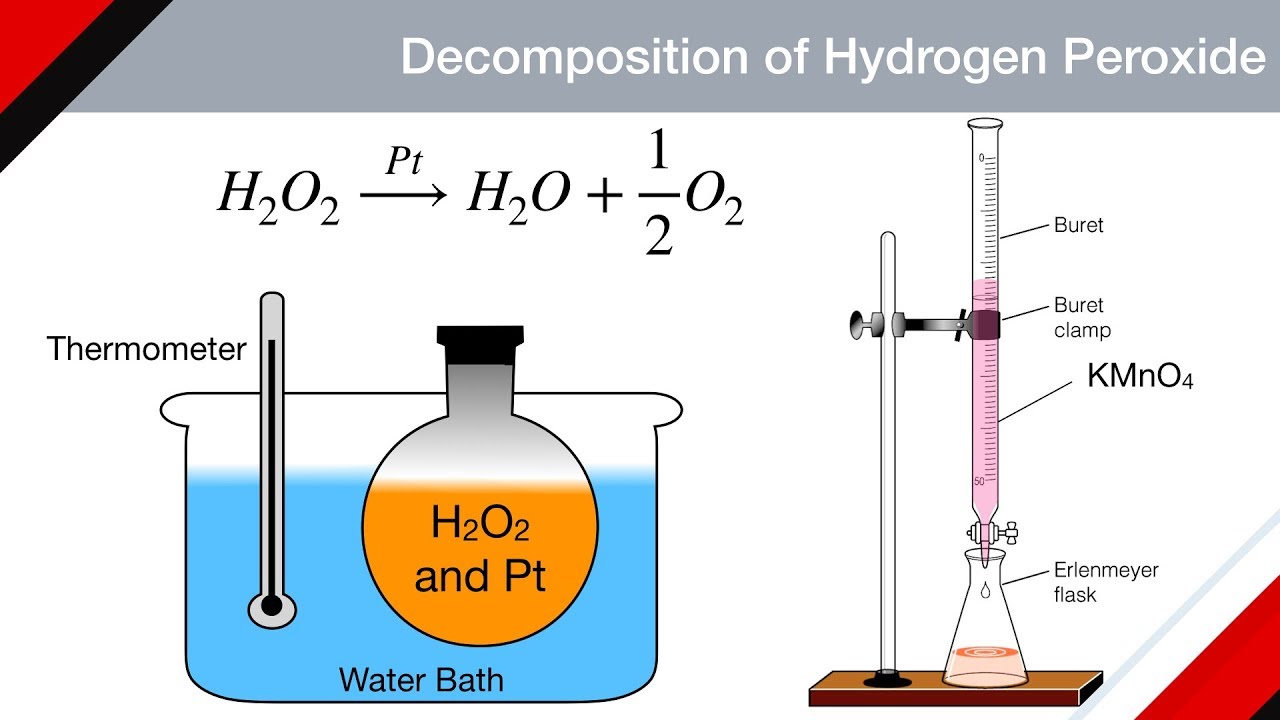
Decomposition of Hydrogen Peroxide Equation
The oxygen bubbles will not burn. The chemical formula for hydrogen peroxide is H 2 O 2.

Multimedia A Catalyst And The Rate Of Reaction Chapter 6 Lesson 5 Middle School Chemistry
Copper reacts with hydrogen peroxide to form water and copper ions plus releasing Oxygen.
. The decomposition of hydrogen peroxide into water and oxygen happens in the presence of light. 2 H 2 O 2 2 H 2 O O 2 g The bubbles formed during the decomposition of peroxide come from oxygen gas. 2 H 2 O 2 2 H 2 O O 2.
Hydrogen peroxide H 2 O 2 is a ubiquitous molecule in nature that shapes the redox state of planetary surfacesGiven that H 2 O 2 is a major oxidant isotope effects associated with H 2 O 2 chemistry play a key role in determining triple oxygen isotopic compositions δ 17 O and δ 18 O of secondary aerosols and minerals which are powerful proxies for understanding. Concentration of H 2 O 2. Chlorine is a chemical element with the symbol Cl and atomic number 17.
It is 100 percent degradable compound. Over 99 of the worlds hydrogen peroxide is manufactured by an autoxidation processWhile the overall equation for this process is fairly simple H 2 O 2- H 2 O 2 there is obviously a lot more to it than that. Germany had very active rocket development before and during World War II both for the strategic V-2 rocket and other missiles.
You can test which gas is hydrogen by lighting a match or lighter over the container. Organic peroxides are organic compounds containing the peroxide functional group ROOR. Among the elements it has the.
Combine equal parts distilled water and 3 hydrogen peroxide in a mixing bowl. We were able to determine our activation energy by manipulating the Arrhenius equation around to be in the form of y mx b. It is acidic in nature and PH is about 45.
Other reactions involving decomposition do require the input of. The one with more bubbles is giving off pure hydrogen. If Hydrogen Peroxide levels are too high then consider inserting copper metal.
If the R is hydrogen the compounds are called hydroperoxides which are discussed in that article. The breakdown of hydrogen peroxide to water and oxygen as well as the breakdown of water to hydrogen and oxygen are examples of decomposition reactions. We own and operate 500 peer-reviewed clinical medical life sciences engineering and management journals and hosts 3000 scholarly conferences per year in the fields of clinical medical pharmaceutical life sciences business engineering and technology.
Decomposition reactions are the breakdown of chemical species into simpler. AB A B is a general equation that can be used to represent this. Similar to what happened in the adult version of Exploding Toothpaste the yeast works as a catalyst to release the oxygen molecules from the hydrogen peroxide solution.
Structure of Hydrogen Peroxide. The hydrogen bubbles will burn. Preparation of H 2 O 2.
2 HAnother example of this type of reaction is the spontaneous decomposition of hydrogen peroxide into water and oxygen. Peresters are the peroxy analog of esters and have general structure RCOOOR. The V-2 used an alcoholLOX liquid-propellant engine with hydrogen peroxide to drive the fuel pumps.
Another issue is hydrogen peroxides rapid decomposition in water and the presence of oxygen radicals. Either from light heat or electricity most of the decomposition reactions require energy. Chlorine is a yellow-green gas at room temperature.
Equation for the Reaction Between Baking Soda and Vinegar. We are an Open Access publisher and international conference Organizer. CoN4 single-atom catalysts SAC have sparkled attention for being highly active in both 2e ORR leading to H2O2 and 4e ORR in which H2O is the main product.
Collect the hydrogen gas by inverting a water-filled tube or jar over the wire producing the hydrogen gas. The rate law depends only on the concentration of H2O2. These same data will be used for questions 6 7 and 8 2 H2O2 --- 2 H2O O2 t seconds o H2O2 M 0882 0697 0566 0458 0372 0236 0188 0094 60 120 180 240 360 420 600 What is the.
It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species ROS. The OO bond of peroxides easily breaks producing free radicals of the form RO the dot represents an. The second-lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them.
Get 247 customer support help when you place a homework help service order with us. Which is decomposition reaction. Hydrogen Peroxide Uses for Plants.
The alcohol was mixed with water for engine cooling. In a chemical equation the left-hand side will represent the reactants and the right-hand side will represent the products of the reaction. It is an extremely reactive element and a strong oxidising agent.
Please contact Savvas Learning Company for product support. It is often referred to as water with one more oxygen atom. The other bubbles are impure oxygen.
Cus H2O2aq 2 Haq Cu2aq 2 H2Ol In the presence of muratic acide I expect youll also notice bubbles forming on the surface of the copper. Ordinarily the reaction proceeds too slowly to be perceived but when you pour hydrogen peroxide. This reaction is one of the exceptions to the endothermic nature of decomposition reactions.
If youve poured hydrogen peroxide onto a cut and didnt get the fizz it is now water. Once a week or after it rains water mature plants with the hydrogen peroxide solution. First be sure to count all of H and O atoms on each side of the chemical equat.
The oxygen-filled bubbles which make up the foam are actually the remainder of what happens when the hydrogen peroxide breaks down into water H 2 O and oxygen O 2. Hydrogen peroxide H 2 O 2 is an important green oxidant 1 widely used in a variety of industries and a promising clean fuel for jet car and rockets 234567 60 wt H 2 O 2 has an energy. An example of a spontaneous without addition of an external energy source decomposition is that of hydrogen peroxide which slowly decomposes into water and oxygen see video at right.
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that through cellular respiration can later be released to fuel the organisms activitiesSome of this chemical energy is stored in carbohydrate molecules such as sugars and starches which are synthesized from carbon dioxide and water hence the name. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. Both Germany and the United States developed reusable liquid-propellant rocket engines that.
2H 2 O 2aq- 2H 2 O l O 2g. Hydrogen peroxide is made through the catalysis of the reaction of hydrogen H 2 with atmospheric oxygen O 2 to produce hydrogen peroxide H 2. A decomposition reaction is a type of chemical reaction in which one reactant yields two or more products.
This form is where lnk is y 1T is x. Catalase has one of the highest turnover numbers of all enzymes. The chemical equation for this reaction is.
Electrosynthesis of hydrogen peroxide H2O2 through oxygen reduction reaction ORR is an environment-friendly and sustainable route for obtaining a fundamental product in the chemical industry. Data for the decomposition of hydrogen peroxide at some set temperature T is provided below. In order to balance H2O2 O2 H2O youll need to watch out for two things.
The decomposition of hydrogen peroxide by itself is. Catalase is a common enzyme found in nearly all living organisms exposed to oxygen such as bacteria plants and animals which catalyzes the decomposition of hydrogen peroxide to water and oxygen.
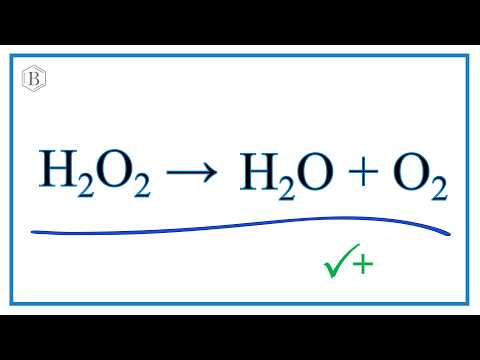
How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube
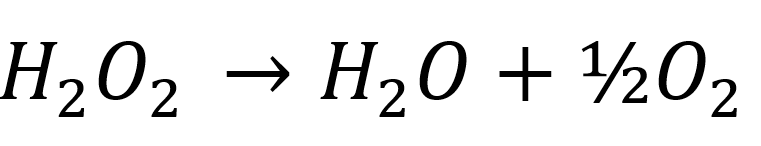
Decomposition Performance Of Hydrogen Peroxide For Use In Bi Propellant Thrusters By Themagicnacho Medium

How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube

Kinetics Of Decomposition Of Hydrogen Peroxide Chemical Kinetics Physical Chem Youtube
0 Response to "Decomposition of Hydrogen Peroxide Equation"
Post a Comment